Photocatalytic Synthesis of Substituted 2-Aryl Morpholines via Diastereoselective Annulation
imcn | Louvain-la-Neuve
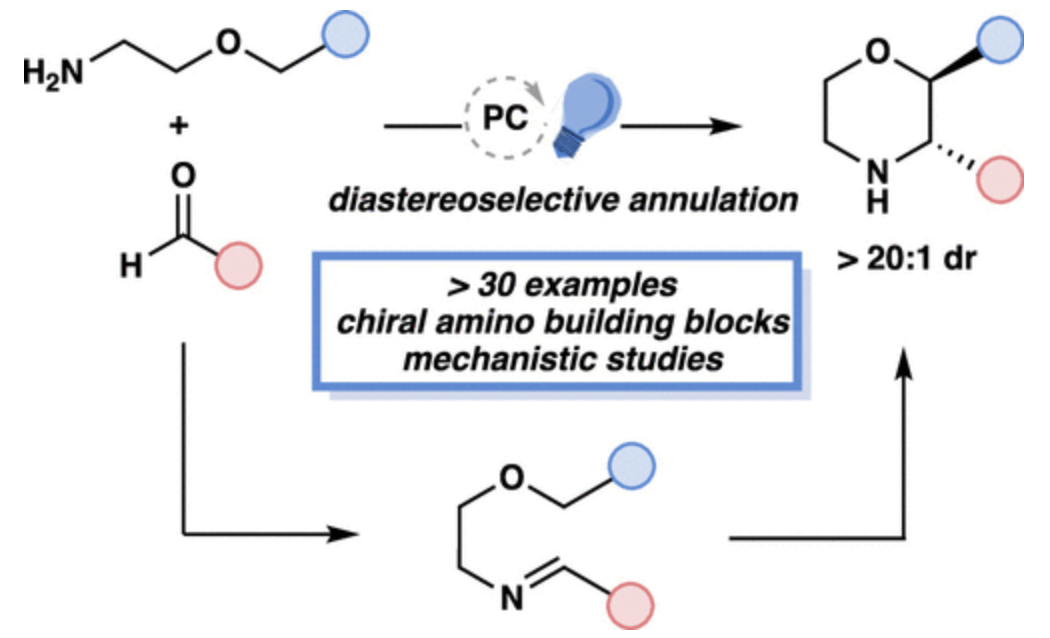
Morpholines are prevalent in medicinal chemistry due to their favorable pharmacokinetic properties and widespread presence in FDA-approved drugs. Existing methods for morpholine synthesis often require prefunctionalized or protected reagents, limiting their versatility and efficiency. Here, we present a photocatalytic, diastereoselective annulation strategy for the synthesis of morpholines directly from readily available starting materials. This method employs a visible-light-activated photocatalyst, Lewis acid, and Brønsted acid to achieve high yields and stereoselectivity. It also provides access to diverse substitution patterns, including challenging tri- and tetra-substituted morpholines. Mechanistic studies reveal that the reaction proceeds through the formation of a radical cation intermediate, with triflic acid playing critical roles in protonating the substrate, preserving the photocatalyst, and preventing product oxidation. Beyond morpholines, this strategy is extended to piperidines, pyrrolidines, and other privileged nitrogen heterocycles. Our findings provide a modular approach for constructing complex, medicinally valuable scaffolds, advancing both synthetic and medicinal chemistry.
Authors: Tiffany A. Brisco, Simon De Kreijger, Vaishnavi N. Nair, Ludovic Troian-Gautier, Uttam K. Tambar