Two Birds, One Stone: Microsecond Dark Excited-State Lifetime and Large Cage Escape Yield Afforded by an Iron−Anthracene Molecular Dyad
imcn | Louvain-la-Neuve
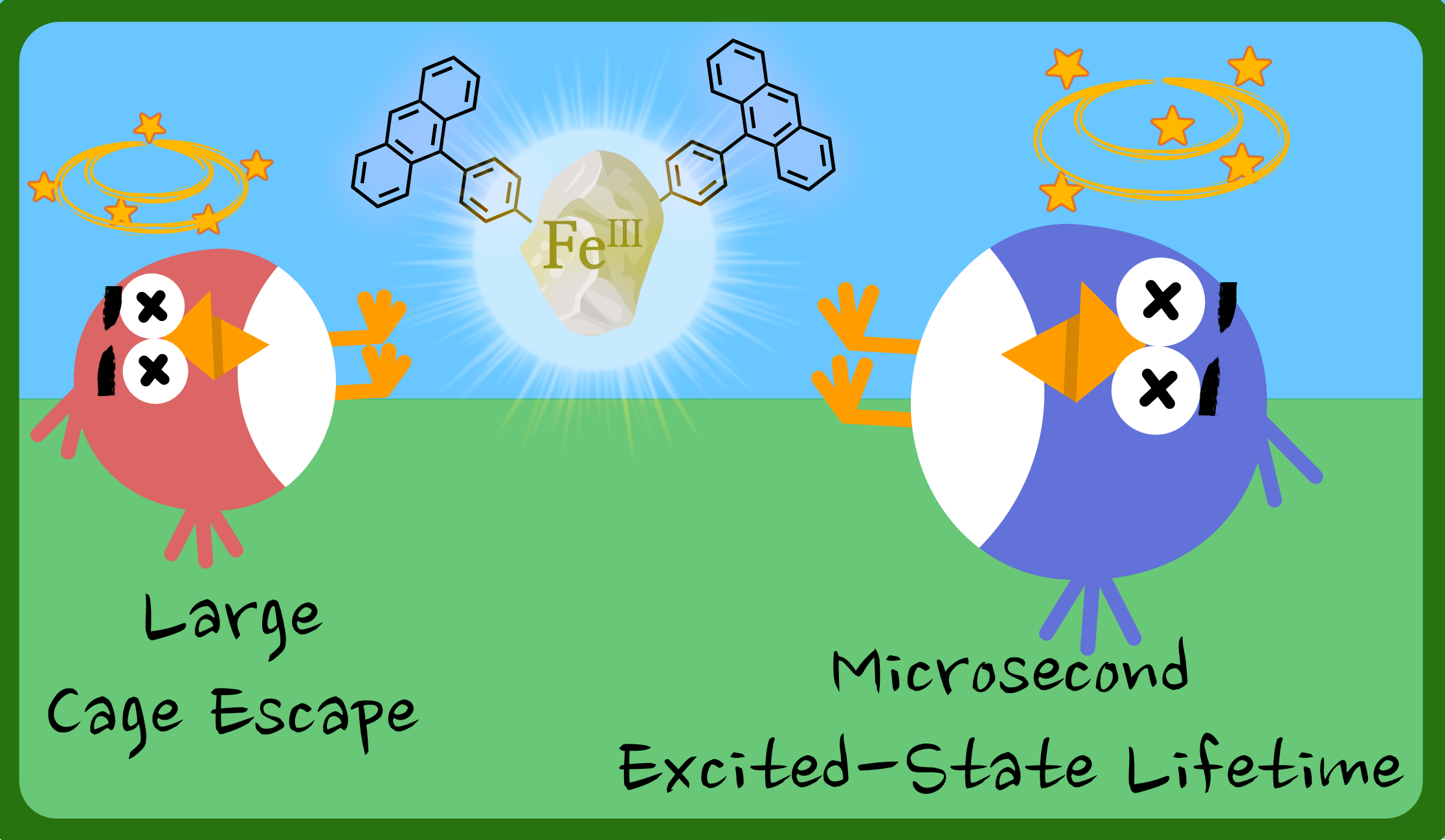
Iron photosensitizers represent a holy grail in photochemistry, but their widespread implementation is limited by their short excited-state lifetimes and poor cage escape yields. Here, the introduction of an anthracene moiety appended to an iron(III) complex allowed to solve both limitations and generate a novel dyad exhibiting an extraordinary excited-state lifetime of 11.5 μs. The key to achieving this remarkably long lifetime is the depopulation of the short-lived iron-centered emissive excited state to populate a dark triplet state located on the anthracene-like moiety with a spin-forbidden deactivation. Population of the triplet anthracene only occurs with ∼10% efficiency in acetonitrile but still allows expansion of the scope of reactivity accessible with iron-based photosensitizers, which now encompasses energy transfer to 3O2. In addition, in a proof-of-principle investigation with methyl viologen as electron acceptor, the population of a triplet excited state allows a drastic ten-fold increase of the cage escape yield from 4.5% for the unsubstituted complex to 42% for the molecular dyad. Hence, this new iron-based dyad provides a complementary approach for complexes based on first-row transition metals as alternatives to well-established analogues based on precious metals. We believe that further spectroscopic investigations and synthetic modifications of the energy acceptor and linkage to the photosensitizer will be key to use these innovative dyads for applications in the near future.
Authors: Felix Glaser, Simon De Kreijger and Ludovic Troian-Gautier